Building nuclear reactor at home - from scratch
 Some time ago I've published article about homemade cpu's, and today we'll be talking about more complex and dangerous things (especially in spite of recent Fukushima accident) – building nuclear reactor at home, which would be able to generate electricity. And before you will start worrying or being skeptical in advance (see Radioactive boyscout) I will say that everything mentioned in this article is more or less safe (at least, as safe as working with Hydrofluoric acid at home), so I strongly recommend anyone to not try this at home. Also, before thinking of doing something – talk to your lawyer – laws are different country-to-country, and many are already in prison
Some time ago I've published article about homemade cpu's, and today we'll be talking about more complex and dangerous things (especially in spite of recent Fukushima accident) – building nuclear reactor at home, which would be able to generate electricity. And before you will start worrying or being skeptical in advance (see Radioactive boyscout) I will say that everything mentioned in this article is more or less safe (at least, as safe as working with Hydrofluoric acid at home), so I strongly recommend anyone to not try this at home. Also, before thinking of doing something – talk to your lawyer – laws are different country-to-country, and many are already in prison 
What are the ways of building nuclear reactor at home?
Nuclear fusion
Heavy Hydrogen (deuterium) is comparably easy to obtain at home – you just need multistage electrolysis of tap water. But there is a tiny problem – even top-end scientists still unable to build reactor which would produce electricity via fusion. So, I guess this way is not feasible for now.Nuclear fission
In the simplest case you just need raw uranium (without any enrichment), and some water (as it's both neutron reflector & moderator). The only problem is that this way you need several hundreds of tons of uranium - and you are not going to get that much without getting too much attention
Are we stuck? There is no way out? Let's take a look at the sky! There are a lot of spaceships, using radioisotope thermoelectric generators – basically they convert energy of passive radioactive decay into usable electricity (BTW that's the major physical cause of the Fukushima problems – even if you shut down reactor, it will generate 7% as much heat at the first minutes, 1% during first weeks, and they will slowly go down to 0.1% of thermal energy generation. I.e. if you have 700MW reactor unit, you will have to dissipate 7MW of heat somehow in the first weeks)
Let's think deeper in this direction. There are 3 major types of radioactive decay:
Gamma-decay
 Sources of gamma radiation are widely used in medical and industrial applications. Most of them are based on Cobalt-60 or Cesium-137 (sadly known after these nuclear disasters). The problem is that gamma radiation is very dangerous, and very hard to shield – 1cm of steel won't save you (see this funny Cherenkov's glow at the right). So, let's try to avoid there. Also, every year lots of guys get free food in jail for trying to sell (or buy) gamma-sources illegally
Sources of gamma radiation are widely used in medical and industrial applications. Most of them are based on Cobalt-60 or Cesium-137 (sadly known after these nuclear disasters). The problem is that gamma radiation is very dangerous, and very hard to shield – 1cm of steel won't save you (see this funny Cherenkov's glow at the right). So, let's try to avoid there. Also, every year lots of guys get free food in jail for trying to sell (or buy) gamma-sources illegally 
PS.If you want to be precise,Co-60 and Cs-137 does not directly emit gamma radiation – it's their short-lived decay-products.
Alpha-decay
Sources of alpha-radiation are widely used in smoke detectors, spark generators, some gas-filled radio-tubes. Ones of the most known isotopes – Americium -241. Alpha-radiation is very easy to shield – just a sheet of paper will stop it, but it's very very dangerous if inhaled or eaten (See the myth of Bloody KGB killing Litvinenko). Anyway, getting significant amounts (over few micro-grams) of alpha-emitters is very hard, so it looks like this way is also not feasible. That's so sad…. The most efficient thermoelectric generators are based on alpha emitters, especially Plutonium-238 (not 239!) – it gives you 0.5 Watt of power per gram, half-life – 87 years (and it costs about 1 megabucks per kilogram).Beta-decay
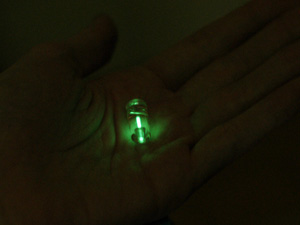
Beta radiation (basically,electrons/positrons) are quite easily to shield, and have very useful property – it electron hits molecule of phosphor – it will emit light. Also, it most countries ‘safe' beta-emitters are quite legal for general public, so we can simple go and buy these multicolored glow key chains. So, it looks like beta-decaying isotopes are our best bet on building homemade nuclear reactor.The core of our reactor – tritium capsule, bought from well-known DealExtreme(but there are lots of shops selling these) - http://www.dealextreme.com/p/mini-tritium-glowring-keychain-10-year-green-glow-6830 . 9.7$. Formally radioactive items are prohibited to be sent by mail, but apparently dealextreme does not know about it

Safety
Low-energy beta radiation cannot get out of capsule casing, and helium is not radioactive. If one brakes the capsule and accidentally inhale tritium – irradiation would be minimal, as human body does not ‘use' pure hydrogen. But if it get burn into water, it might end up being part of your body cells, and you will get maximum possible radiation over next few decades. So, don't break, don't burn and don't inhale it
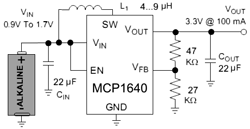 So, Tritium is a ‘superheavy' hydrogen, it has half-life of 12.32 years. It's decay products are Helium, electron (6.5kEv, and for picky readers, an antineutrino). We'll gather energy with solar cell, fed up into MCP1640 step-up boost converter (works down to few tens of volt at the input), will charge 1F/5V EDLC (supercapacitor) and an red led as the load.
So, Tritium is a ‘superheavy' hydrogen, it has half-life of 12.32 years. It's decay products are Helium, electron (6.5kEv, and for picky readers, an antineutrino). We'll gather energy with solar cell, fed up into MCP1640 step-up boost converter (works down to few tens of volt at the input), will charge 1F/5V EDLC (supercapacitor) and an red led as the load. In order to gather as much light as possible, I put capsule into reflector made of aluminum foil + cover everything with foil from the inside.
In order to gather as much light as possible, I put capsule into reflector made of aluminum foil + cover everything with foil from the inside.  In order to focus light on solar cell I use 2x 10 diopter lenses . On the photo you see solar cell before gluing, tritium capsule is not set up.
In order to focus light on solar cell I use 2x 10 diopter lenses . On the photo you see solar cell before gluing, tritium capsule is not set up.Connect it all, turn off the lights, wait a minute or two to charge supercap, and here is the result:

Is it "Free energy"?
Surely not :-) This reactor produces just about 7mW of power (and after 12.32 years it will be 3.5 ), so while it is ok for a LED, it's way too low to charge your notebook . From the other side, 10 of these modules can power cell phone in stand-by for several decades :-)
), so while it is ok for a LED, it's way too low to charge your notebook . From the other side, 10 of these modules can power cell phone in stand-by for several decades :-) Here goes price calculation: capsule :9.7$, solar cell : 5$, lenses 13.8$*2 = 42$ per module. For 10 of these you will need to fork out 420$... From the other side, there are bigger capsules - for 35$ - maybe they will be more cost-efficient.
Comments/questions/opinions – are welcome :-)




 @BarsMonster
@BarsMonster