Fuming nitric acid synthesis
It is not that easy to buy 100% nitric acid due to it's instability (and hence special requirements for transportation) and wide range of it's applications. Distillation does not allow one to get above 68%. If we cannot live without 100% - we'll have to synthesize it ourselves. Last time I did that using quite inconvenient and unsafe method (decomposition of lead(II) nitrate, then dissolving mix of liquid nitrous oxides in 68% nitric acid. This process was too scary to describe in detail
This time I went for conventional and relatively safer route: potassium nitrate (100g) + 98% sulfuric acid (125ml, ~50% excess). You can then distill nearly 100% pure nitric acid.
Final result was reached much easier and faster. Yield was 50ml (which is ~50%, had no more time and ice to continue the process). Next time I will ether have to try something more high-tech (like Peltier cooling with Liebig condenser - Graham's condenser seems to be an overkill and strictly speaking you cannot use it at this angle), or run the process at winter (but it's probably not a good idea to store >100ml of 100% nitric acid due to it's instability).
I've tried a bit of final product right away by etching Texas Instruments IC with very chemically durable plastic.

Conical flask with potassium nitrate and sulfuric acid with glued thermal probe:



It is hard to keep cooling water under 21°C at summer (so that remaining NO2 does not evaporate), so I had add ice blocks pre-cooled to -35°C from time to time:

Receiving flask also had to be cooled:

Here you can see that when Graham's condenser is installed at an angle liquid is trapped in the coils which causes non-continuous liquid flow - that is why it is not recommended to install condenser this way.






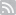
 @BarsMonster
@BarsMonster